Thursday, August 29, 2013
Ethylene glycol diglycidyl ether of some of the information
Appearance: light yellow or colorless transparent liquid.
Solubility: soluble in ethanol, acetone and benzene and other organic solvents, slightly soluble in water.
Description: Ethylene glycol diglycidyl ether is a glycol ether chain containing an epoxy resin, an epoxy resin or a low viscosity water-soluble aliphatic epoxy resin, epoxy equivalent 112 ~ 135g/eq or an epoxy group content of 28.5% to 33. O%, viscosity of 10 ~ 100mPa · s, the chlorine content of 9.5%. Soluble rate of 95% to 100%.
Preparation: ethylene glycol and epichlorohydrin.
Usage: often mixed with bisphenol A type epoxy resin as a low viscosity compound, casting material, impregnating liquid, adhesive, resin modifier, fabric treatment agents. Are used as epoxy reactive diluents, stabilizers paraffin.
More about:Ethylene glycol diglycidyl ether suppliers
From:chemical reagent
Thursday, August 22, 2013
2,5-Dithiobiurea description of each parameter
Preparation:
Method one: The preparation method is the reaction vessel with stirring hydrazine sulfate into wet basis, a small amount of catalyst is added, and then into ammonium thiocyanate, open jacket steam heating under reflux 3.5h, cooled, discharge, filtered, washed with water , vacuum filter to dryness to give the finished product.
Method Two: Hydrazine sulfate and thiocyanate amines by condensation, heavy discharge, too.
Storage features: Treasury ventilated low-temperature drying; oxidants, acids stored separately
Toxicity rating: high toxicity
Acute toxicity: Oral - Rat LD50: 500 mg / kg; celiac - Mouse LD50: 100 mg / kg
Health Hazard: heat decomposition of toxic emissions of nitrogen oxides and sulfur oxides
Category: Pesticides
Extinguishing Media: Water, dry chemical, dry sand, carbon dioxide, foam, extinguishing agent 1211
Use one: Is 2,5-Dithiobiurea leaf blight fungicide intermediates.
Uses two: pesticide and pharmaceutical intermediates.
Appearance: Bisthiourea pure product as a white crystalline solid, mp206 ~ 208 ℃, in hot water with some solubility, soluble in pyridine, triethylamine, dimethylformamide and general solvent.
More about:2,5-Dithiobiurea suppliers
From:chemical reagent
Tuesday, August 13, 2013
Aluminum tert-butylate basic information
Chinese Name: tert-butoxy aluminum
Chinese Synonyms: tert-butoxide
English name: aluminium tri-tert-butanolate
English Synonyms: Aluminum tert-butoxide; Aluminum tert-butanolate; Aluminum tert-butylate; Aluminum tri-tert-butoxide; Aluminum, tri-tert-butoxy-; Aluminum, tris-tert-butoxy-; NSC 4648; Tri-tert- butoxyaluminum; 2-Propanol, 2-methyl-, aluminum salt; 2-Propanol, 2-methyl-, aluminum salt (3:1); Aluminium tri-tert-butanolate; tert-Butyl alcohol, aluminum salt (8CI); Aluminum t-butoxide; aluminum tris (2-methylpropan-2-olate)
CAS :556-91-2
EINECS :209-146-1
Formula: C4H10AlO
Molecular Weight: 101.1015
More about:Aluminum tert-butylate suppliers
From:chemical reagent
Monday, August 12, 2013
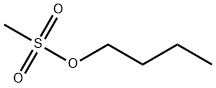
Butyl methanesulfonate some information
Chinese name: methyl sulfonate ester
Chinese Synonyms: methyl sulfonate ester; butyl mesylate
Product Name: N / A
English Synonyms: Butyl methanesulfonate
CAS No.: 1912-32-9
Formula: C5H12O2S
Mol File: Mol File
Description: This product is colorless transparant liquid.It is soluble in alcohol.It is toxic.It is used for organic synthesis.This product should be sealed.
More about:Butyl methanesulfonate suppliers
From:chemical reagent
Tuesday, August 6, 2013
N-Bromosuccinimide chemical reactions related
N-Bromosuccinimide reaction of olefins
NBS can olefin 1 in aqueous solution reaction of hydroxy alkyl bromide 2. The optimal reaction condition is at 0 ° C under an olefin dissolved in dimethyl sulfoxide, dimethoxyethane, tetrahydrofuran, tert-butanol, 50% aqueous solution of any one of the segments joined NBS. The reaction mechanism is: i) an onium bromide ion generation; ii) the nucleophilic attack of water molecules. Stereoselectivity for the trans-adduct in accordance with the law Markov.
The products of the reaction include the α-bromo ketone and two bromo compound. The new NBS purified by recrystallization can reduce the formation of these by-products. If no water was added, and the addition of other nucleophiles may bifunctional synthesis of other compounds.
Hofmann rearrangement reaction of bromide
N-bromosuccinimide in a strong base (e.g., 1,8 - diazabicyclo [5.4.0] undec-7 - ene (DBU)) state can be re-primary amide by Hofmann discharge reaction intermediate isocyanates. The isocyanate compound and a hydroxyl group (e.g., methanol) to form a readily hydrolyzable carbamate and separation.
More about:N-Bromosuccinimide suppliers
From:chemical reagent
Sunday, August 4, 2013
Some properties of 1,4-Butanesultoned
Chemical Properties: colorless liquid. Mp 12.5-14.5 ℃, boiling point 134-136 ℃ (0.53kPa), the relative density of 1.331 (20/4 ℃), refractive index 1.4640, with a variety of organic solvents, insoluble in water.
Uses: medicine, chemical reagents, surface active agents, dye-sensitized colored intermediates. Electroplating intermediates starting materials. Sulfonation agent for organic fine chemical intermediates for the pharmaceutical, photographic materials, lithium batteries, electronic plating, household chemicals, etc. used as photosensitive 67649
Production methods: by tetrahydrofuran and ethyl chloride chlorine butyl acetate, and then reacted with sodium sulfite hydroxybutyric acid, dehydration in the final product. About 40% overall yield.
Category: toxic substances
Toxicity rating: high toxicity
Acute toxicity: Oral - Rat LD50: 500 mg / kg; celiac - Mouse LD50: 138 mg / kg
Health Hazard: Thermal decomposition emits toxic fumes of sulfur oxides
Storage features: Treasury ventilated low-temperature drying; kept separate from raw materials and food
Extinguishing Media: Water, dry chemical, dry sand, carbon dioxide, foam, extinguishing agent 1211
More about:1,4-Butanesultone suppliers
From:chemical reagent
Thursday, August 1, 2013
Methyl caprylate some information about each parameter
Methyl caprylate is a colorless to pale yellow clear liquid. With wine, fruit, orange aroma. And difficult to dissolve in water, soluble in alcohol, ether, oils and other organic solvents. Naturally present in the iris and creamy strawberry, pineapple, plums and other essential oils. Preparation methods generally two types: one, from coconut oil fatty acids in the presence of an alcohol solution of hydrochloric acid gas derived. 2, by the bitterness esterification with methanol derived. Colorless, oily liquid. Mainly for the preparation of pineapple, berries and fruit flavor.
More about:Methyl caprylate Exporters
From:Natural fatty acids
Subscribe to:
Posts (Atom)